|
The most recent general review of the Castniidae, focusing on the
Neotropical Castniinae, is that of Miller (1986[9]), though shorter accounts of
the family may be found in Holloway, Bradley & Carter (1987), Common (1990)
and Scoble (1992). Miller placed S.E. Asian castniids in a subfamily Tascininae,
sister to the Castniinae which include the diverse neotropical representation as
sister to the Australian ones (as subtribes Castniidi and Synemonidi of tribe
Castniini, equivalent to the Castniinae). Hampson (1895) erected the family
Neocastniidae for the Oriental taxa but later (Hampson, 1918) changed this to
Tascinidae without comment. The former family-group name should strictly have
priority. Heppner (1993[6]) recognised three subfamilies: Castniinae,
Synemoninae and Neocastniinae. Edwards et al. (in press) recognise two:
Castniinae, with tribes Castniini and Synemonini; Tascininae. This last
arrangement is followed here.
All castniids are robust with clubbed antennae and are day-flying,
usually brightly coloured. The antennal club usually has an apiculus. The
Australian taxa are somewhat more delicate than the rest, with wings somewhat
less deep. All have a rather characteristic venation with the M-stem present in
the cells of both fore- and hindwings, and very much closer to CuA than to Rs,
forming a narrow sub-cell that gives rise to M2, M3, CuA1 and CuA2 in a
quadrifid arrangement (Holloway, 1986a, Fig 1).
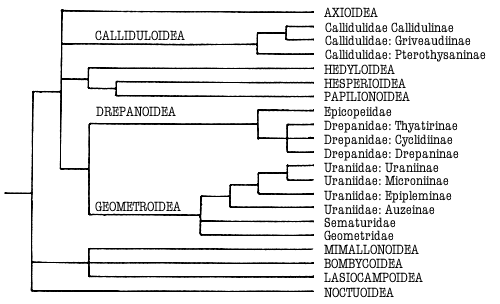
Figure 1. Phylogeny of the Macrolepidoptera as suggested by Minet
(1991) and Kristensen & Skalski (in press). Superfamilies are indicated in
capitals. Groups treated in this work are indicated in bold.
The forewing usually has an areole within the radial system in the
Castniinae but not in the Tascininae. The terminal bifurcation of the radial
system is R3 + R4 in the Castniini, R4 + R5 in the Synemonini and in the
Tascininae. The Tascininae have lost discocellular cross-veins in fore- and
hindwings, including that between M and CuA in the latter: CuP is also absent
from both. The Tascininae have an unusual branching system for the forewing
radial veins and M1: R1, (R2, R3), ((R4, R5) M1) in typical Tascina Westwood
with (R1 (R2, R3)) in the type species of Neocastnia Hampson. A chorda is
sometimes present. The frenulum is strongly developed. The tongue is present in
the Castniinae, absent in the Tascininae. Ocelli are present but chaetosemata
are absent.
The male genitalia in the Castniinae have the uncus divided or
bifurcate, but generally variable in structure. The gnathus is present. The
saccus is broadly and sharply bifurcate. The aedeagus is recurved, more strongly
so in Neotropical taxa. The valve is broad but simple. The genitalia of Tascina
Westwood are described below: the saccus is entire, the aedeagus simple, not
recurved.
In the castniine female the ovipositor and apodemes are elongated, the
lobes of the former sometimes acute, blade-like, for insertion of eggs into
roughened plant surfaces or soil. The development of the ductus bursae varies.
There may be one or two spiny signa in the bursa. The genitalia of the tascinine
female are described below.
The eggs of Castniinae are spindle-shaped, laid flat, with four
longitudinal ribs in Synemonini, five in Castniini, and with numerous transverse
striae between them.
The larva has a prognathous head, partially retracted into the prothorax,
where there is a sclerotised, prothoracic shield. There are small dorsal
sclerotised areas on segments T2 to A2 in Synemonini, and the cuticle is
generally spinulose. Primary setae are in pinacula. The prolegs are short,
broad, with few crochets, uniordinal in two transverse bands: in Castniini these
are progressively lost through succeeding instars, being replaced by numerous
microspicules surrounding a T-shaped ampullar lobe (Miller, 1986[9]). Miller
gave details of larval chaetotaxy and noted that later instars of Castniini
produce a reddish brown exudate from a prothoracic gland, and secondary setae
develop in later instars of Synemonini.
In the Neotropics the larval hosts are monocotyledons in the families
Bromeliaceae, Gramineae, Marantaceae, Musaceae, Orchidaceae and Palmae (Miller
1986[9]; Edwards et al., in press). The larvae feed endogenously,
excavating a tunnel within the host, e.g. in a palm leaf rachis. The Australian
Synemonini feed initially in the tillers and butt of their host-plants (Cyperaceae,
Ecdeiocoleaceae, Gramineae and Lomandraceae), with later instars feeding on
rhizomes from a silk-lined tunnel in the soil: pupation occurs in a chimney
extending upwards from this tunnel (Common, 1990). The life cycle in Synemonini
requires at least two years but is shorter in Castniini. Nothing is known of the
biology of Tascininae: even the adults are extremely rare, with specimens in
collections probably numbering no more than about twenty.
Miller discussed the biogeography of the family, suggesting it
established its current range prior to the break-up of Gondawanaland, and was
possibly well represented in Antarctica at that time. The vicariance of the
Tascininae is unclear: they could have separated from the Castniinae with India
in the late (120Ma) Cretaceous (Hall, 1998), subsequently following more humid
tropical conditions to Sundaland, or they could have separated earlier on
Gondwanan components of South-east Asia, perhaps in the late Jurassic
(separating at about 165Ma) and early Cretaceous on continental terranes now
attached to western Burma and (Woyla terranes) Sumatra (Metcalf, 1996, 1998).
The Gondwanan biogeography of the Castniidae is also reviewed by Holloway &
Hall (1998).
The higher classificatory position is still subject to some
disagreement. Common (1990), Scoble (1992), Heppner (1993[6]) and Nielsen,
Edwards & Rangsi (1996) place the family on its own in the superfamily
Castnioidea, whereas Minet (1986, 1991) included the family in the Sesioidea on
the basis of three apomorphic features: the ocular diaphragm is more heavily
pigmented anteriorly; large patagia; the condition of the metafurcal apophyses.
This latter arrangement is supported by Edwards et al. (in press).
There is more general agreement about the relationship of the Sesioidea
to the Cossoidea, possibly with the Zygaenoidea more distantly related (e.g.
Minet, 1991; Edwards et al., in press). Miller (1986 [9]) noted the
condition of the basal abdominal tergites, including an ovoid, scaleless area on
each side of the second tergite, as being shared with the Cossidae, and she
suggested general larval and pupal features also supported this affinity. Minet
(1991) referred to two specific larval features: an oblique dark stripe between
the dorsal setae of the larval pronotum; irregularly distributed spinules on the
integument.
Scoble (1992) noted the presence of flavonoid pigments in Castniidae in
common with sphingids, noctuids and butterflies, but did not indicate this to be
of any higher classificatory significance.
>>Forward
<<Return to Contents page
|

